About us
From the project’s inception to the creation of ICOVELL
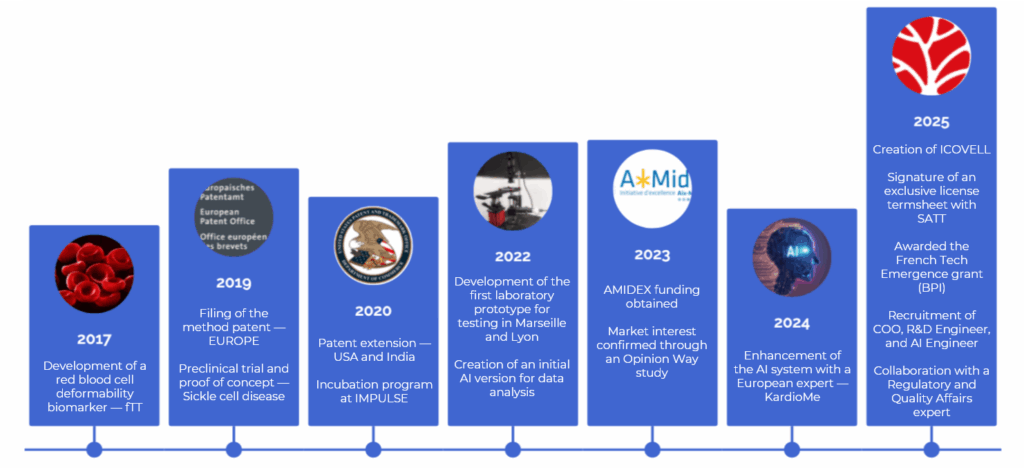
ICOVELL was born from an academic research project launched in 2014 by a team of CNRS scientists, focusing on the deformability of red blood cells.
The company was officially established in 2025.
Our team

Annie Viallat, PhD
With over 20 years of experience, she has earned an international reputation for her pioneering work on the microflow dynamics of blood cells and biofluids, as well as in advanced fast video microscopy techniques. A recognized expert in her field, her leadership plays a pivotal role in driving the project forward.
CEO & Co-founder

Aurélien Valet
With over 20 years of experience in the MedTech industry, he brings a deep understanding of the challenges of industrialization, product development, and market launch. His hands-on expertise is key to structuring the company and accelerating its growth.
COO & Co-founder
Catherine Badens, PhD, Pharma D
Head of the Biochemistry Department at La Timone Hospital and lecturer at the Faculty of Pharmacy, she is a leading expert in rare red blood cell disorders. Her deep knowledge of these diseases is essential for the clinical validation and medical impact of our device.
Co-founder

Emmanuèle Helfer, PhD
Trained as a physicist, she has deep expertise in biophysics, cell biology, and biocompatibility. Her mastery of in vitro approaches to biological challenges is a key asset for ensuring scientific robustness.
Co-founder

Anne Charrier, PhD
A physicist specializing in bio-interfaces, she is actively involved in developing measurement devices for red blood cell deformability. Her experience in technological development is a valuable asset for the implementation of the device.
Co-founder

Antoine Vian, PhD
He plays a pivotal role in technical development. He oversees the prototyping of the medical device, data analysis and processing, the development of new features, and the formalization of the technical documentation.
R&D Engineer

Montassar Chouikh
His expertise spans the design of intelligent microservices, natural language processing (NLP), automated data extraction, and the implementation of RAG and LLM architectures.
AI Engineer
Partners & supports

She has extensive experience in the development and registration of Class IIb and III medical devices, as well as borderline products, both in Europe (MDR) and the United States (510(k), PMA). She also has strong expertise in defining preclinical and clinical evaluation strategies.
Patricia Forest-Villegas
Regulatory & Quality Consultant

He holds a PhD in cardiac imaging from Inria, Mines ParisTech, and Microsoft Research Cambridge. He has worked at several research institutes, co-founded a start-up specializing in medical imaging, and developed artificial intelligence platforms for healthcare.
Jan Margeta
KardioMe, AI Consultant

Le French POC
Prototyping













